[on July 29,2023]
Visionary NOUGEIKAGAKU 100
Symposium(3) on Natural Product Chemistry Research Area: "Natural Product Chemistry 4.0: Beyond Structure, Synthesis, and Biological Activity"
Date & venue; Tohoku University on July 29,2023
1. Development of plant circadian clock modulators
Junichiro Yamaguchi, Professor, Faculty of Science and Engineering, Waseda University
Since the reproductive behavior of plants depends on the circadian clock, artificially controlling the clock timekeeping system could enable improvement in food production and supply of biomass resources. We successfully identified circadian clock modulators, PHA 767491 and BML-259, to lengthen the circadian rhythm of Arabidopsis thaliana using our developed high-throughput screening. The development of higher active molecules and the discovery of target proteins have enabled further mechanistic elucidation of the plant circadian clock.
2. Development of structure optimization process to accelerate natural product drug discovery
Satoshi Ichikawa, Professor, Faculty of Pharmaceutical Science, Hokkaido University
Natural products are a rich source in drug discovery because they possess important biological activities and structures beyond human knowledge. How to rapidly and systematically synthesize the necessary natural product derivatives?" is a challenge in natural product drug discovery. In this lecture, I will introduce recent our researches including the structural optimization of colistin using the serine-threonine ligation reaction and the structural optimization study of a group of MraY-inhibitory nucleoside natural products using.
3. AI Expands Potential of Molecular Design
Masahito Ohue, Assistant Professor, School of Computing, Tokyo Institute of Technology
AI technology, specifically AlphaFold2, has revolutionized protein structure prediction and attracted widespread attention from researchers in various fields. Its applications extend to areas such as bioinformatics, drug discovery, and molecular design. The development of structural informatics based on AlphaFold2, including protein-protein docking, protein-peptide docking, and ligand superimposing, is progressing rapidly. This talk explores the potential of informatics and AI innovation in biology/chemistry, highlighting the utilization of AlphaFold2 in designing target-binding peptides and antibodies, as well as its impact on small molecule drug discovery. Successful integration with AI technology is crucial for advancing molecular design possibilities.
4. Chemical biology of natural products pioneered by synthesis and isolation.
Midori A. Arai, Professor, Faculty of Science and Technology, Keio University
In our laboratory, we study chemical biology while learning from natural products. I will present about two topics, 1) creation of rocagramid molecule targeting cancer with abnormal expression of transcription factor ASCL1 and 2) activation of cryptic genes and isolation of natural products by co-cultivation of microorganisms and animal cells.
5. Toward forecast and creation of natural products from accumulation of biosynthetic reactions
Tomohisa Kuzuyama, Professor, Graduate School of Agricultural and Life Sciences, The University of Tokyo
We aim to establish a new paradigm for natural product chemistry by making breakthroughs through diverse research spanning a wide range of fields, including bioorganic chemistry, synthetic organic chemistry, synthetic biology, structural biology, theoretical chemistry, information science, artificial intelligence (AI), etc. More specifically, we aim to establish a new paradigm in natural product chemistry; we will lead a fundamental change from the conventional concept, which has persisted for more than half a century in the field of natural product chemistry, that natural products are something to be "searched for," to the idea that natural products are something to be "created.
6. Forty years with PKC ligands – their structural simplification toward new medicinal seeds
Kazuhiro Irie, Professor, Graduate School of Agriculture, Kyoto University
Protein kinase C (PKC) isozymes are potential targets for treating cancer and HIV infection. Although natural PKC ligands have the potential to become therapeutic leads, most of them are potent tumor promoters. We have identified 10-methyl-aplog-1, a simplified analog of tumor-promoting aplysiatoxin, as a possible therapeutic lead for cancer and HIV infection. In contrast, a new scaffold is needed to develop PKC ligands with remarkable isozyme selectivity. Taking advantage of machine-learning and computational chemistry approaches, we screened the PubChem database to select sesterterpenoids alotaketals as potential PKC ligands, then synthesized simplified alotaketal analogs with binding preference for conventional PKC isozymes.
7. Lipid Membranes as Action Targets of Biologically Active Natural Products
Michio Murata, Professor, Graduate School of Science, Osaka University
The antifungal agent amphotericin B is a drug that exhibits pharmacological activity without binding to proteins. This antibiotic forms a specific complex with ergosterol present in fungal cell membranes, forming tubular self-aggregates (barrel-stave type) that penetrates the lipid bilayer and exhibits fungicidal activity through the flux of ions through its pores (Figure 1). For many years, however, this barrel-stave structure has not been experimentally elucidated. Structural studies of the channel assemblies that form only in lipid membranes have been reported only for antimicrobial peptides, but rarely for non-peptidic compounds (so-called natural products). Recently, we have elucidated the structure of this ion channel at the atomic level and obtained clues as to the molecular mechanism of its selective toxicity.
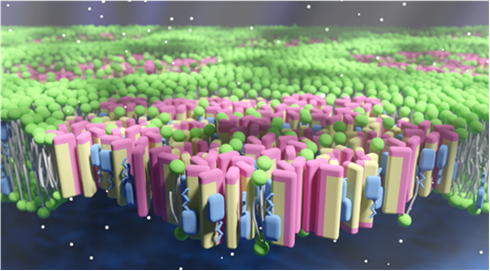
Figure 1: Illustration of putative ion-permeable channels formed by amphotericin B in the fungal membrane.
References
1) Y. Umegawa, T. Yamamoto, M. Dixit, K. Funahashi, S. Seo, Y. Nakagawa, T. Suzuki,
S. Matsuoka, H. Tsuchikawa, S Hanashima, et al.: Sci. Adv., 8, eabo2658 (2022).
2) T. Yamamoto, Y. Umegawa, H. Tsuchikawa, S. Hanashima, N. Matsumori, K. Funahashi,
S. Seo, W. Shinoda & M. Murata: Biochemistry, 58, 5188 (2019).
3) Y. Nakagawa, Y. Umegawa, N. Matsushita, T. Yamamoto, H. Tsuchikawa, S. Hanashima,
T. Oishi, N. Matsumori & M. Murata: Biochemistry, 55, 3392 (2016).












































